Table 2 Synthesis of 3-aryl- and 3-cyclohexyl-4-formylsydnone N-(tetra-O-acetyl-β-d-glucopyranosyl)thiosemicarbazones (4a–o) under conventional and μ-wave heating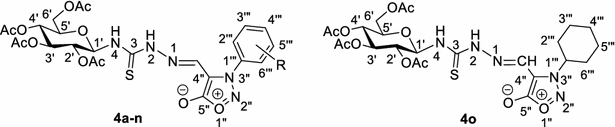
Entry | R | Reaction time (min) | Yield (%) | ||
|---|---|---|---|---|---|
Conventional heating | MW heating | Conventional heating | MW heating | ||
4a | H | 100 | 25 | 50 | 71 |
4b | 2-Me | 120 | 28 | 55 | 75 |
4c | 3-Me | 130 | 30 | 55 | 73 |
4d | 4-Me | 130 | 30 | 56 | 76 |
4e | 2,3-diMe | 130 | 35 | 55 | 70 |
4f | 2,4-diMe | 130 | 35 | 50 | 68 |
4g | 4-Et | 120 | 28 | 60 | 83 |
4h | 3-OMe | 130 | 30 | 60 | 78 |
4i | 4-OMe | 130 | 30 | 60 | 81 |
4j | 4-OEt | 130 | 25 | 60 | 82 |
4k | 4-F | 130 | 30 | 55 | 65 |
4l | 4-Br | 150 | 35 | 55 | 63 |
4m | 4-I | 130 | 35 | 57 | 68 |
4n | 2-Me-5-Cl | 140 | 45 | 50 | 43 |
4o | Cyclohexyla | 130 | 30 | 60 | 85 |
